What will happen when you shake oil with water? Will oil dissolve in water to form a homogenous solution? The answer to this question is an absolute no because we have seen how oil forms a separate layer over the surface of water when we are boiling spaghetti in our kitchen. The same happens when a chemist shakes oil and water in a separatory funnel in his chemistry laboratory. This clearly means that oil and water don’t mix together. Rather, the two are immiscible chemical compounds. What’s the secret behind it? Let’s find that out through this article.
Why don’t oil and water mix?
Like dissolves like. This means for chemical compounds to interact well with each other, they must possess a similar chemical nature. Oil and water don’t mix with each other because of the difference in their polarities. Oil is a non-polar molecule while water (H2O) is extremely polar. There are no oppositely charged regions to interact with polar H2O molecules neither can the oil molecules develop hydrogen bonds with water. It’s really important to know if the given molecule is polar or nonpolar.
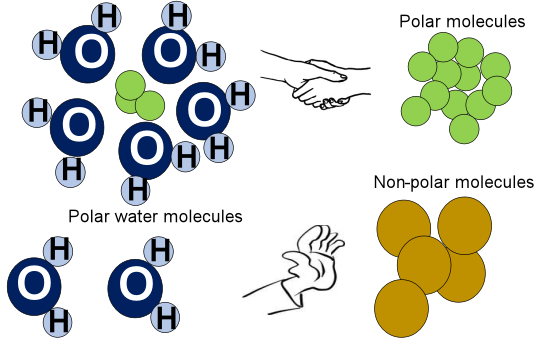
Why are water molecules polar?
A water (H2O) molecule is polar due to the difference in electronegativity between the covalently bonded oxygen (O) and hydrogen (H) atoms present in an O-H bond. Oxygen more strongly pulls the shared O-H electrons. A non-uniform charge distribution develops in the molecule which is further endorsed by its asymmetric bent shape and molecular geometry. Thus, each H2O molecule in water is polar which makes it a polar universal solvent as a whole.
It is a universal solvent because it can dissolve a diverse range of chemical compounds such as ammonia gas (NH3 ), ethanol (CH3CH2OH), methanol (CH3OH), table salt (NaCl), glucose (C6H12O6), etc. But, all these compounds are essentially polar in nature. They use their negative and positive poles to interact with oppositely charged Hδ+ and Oδ- ends of water respectively.
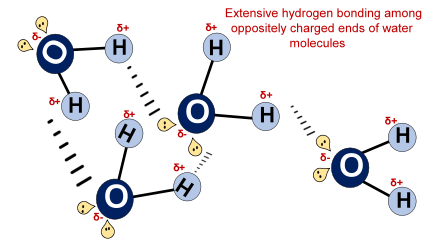
These molecules are highly water-soluble also because of their ability to form H-bonding with water.
However, non-polar molecules such as oil neither have polar ends nor can it form H-bonding with water, so it stays immiscible with water.
Why are oils non-polar in nature?
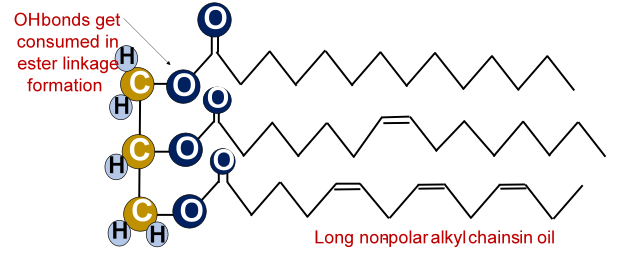
Oils are organic compounds made up of a large number of carbon, hydrogen, and oxygen atoms covalently bonded to each other. Traditionally oils are fatty acid esters for e.g., triglycerides which are made up of covalently linked fatty acids and glycerol molecules. Glycerol is a tri-alcohol, it has 3 OH bonds present but the OH bonds get consumed in chemical bond formation. Thus, the overall oil molecule is non-polar with ester (COO) bonds sandwiched between numerous non-polar alkyl chains.
What happens when we mix oil with water?
Here again, we can take a clue from the like dissolves like concept. When we mix oil with water, similar polarity oil molecules attract each other. Similarly, the water molecules get attracted to their own family members i.e., the other water molecules. Polar water molecules cannot break the bonds present between non-polar oil molecules, so the latter stays dissoluble in the former. In this way, the two chemical compounds form distinct separate layers. As water is denser than oil due to the extensive hydrogen bonding present among H2O molecules, so the oil layer appears above the water layer.
What is the importance of water-oil immiscibility in chemistry?
The water-oil immiscibility factor is often used in chemistry laboratories to separate a mixture into its constituent chemical compounds and the technique is known as liquid-liquid partitioning. Polar organic compounds from a complex sample mixture become a part of the aqueous layer while non-polar molecules are extracted in the oil layer.
Can we mix oil with water through an external force?
The interesting fact is that nothing is impossible when it comes to science and especially chemistry. So, yes we can force water and oil molecules to interact with each other by adding a surfactant or an emulsifier. An emulsifier has both polar and non-polar ends. So, it can interact dually with water and oil molecules. In this way, water although don’t actually mix up (in the literal sense) but interacts to a large extent with oil. This results in an emulsion.
So, the next time someone asks you how you can wash off grease stains from your clothes using water if water and oil do not mix, you know the answer to this question. That is, we can do so by using detergents.
